
We will begin studying new material by solving a crossword puzzle:
- The smallest particle of matter
- Speed \u200b\u200bof movement
- Unit of length
- The phenomenon of maintaining the speed of movement of the body or its rest.
- The value that can be measured using a beaker.
- 6. The value that is measured in pounds, carats, centners.
- The device for measuring temperature.

We will choose several rectangular-sized cast-iron and aluminum ingots of different sizes (see figure). Using scales, we measure the mass of each ingot, and using a ruler, we measure their volumes.
Cast iron ingots
Weight kg
Volume dm 3
their private
Aluminum ingots
When dividing the mass of each ingot by its volume, the same values \u200b\u200bof the quotient are obtained for all cast iron ingots ("7 kg / dm3) and for all aluminum (" 3 kg / dm3). That is, regardless of the specific values \u200b\u200bof mass and volume, their quotient (the result of division) remains a constant value for a given substance. This amazing regularity was the reason for introducing a special quantity into physics - the density of the substance.

A physical quantity showing how much mass of a substance is per unit volume of a substance is called density substances.
Physical quantity
Designation
unit of measurement
m
V
Density
ρ (ro)
Kg / m, g / cm
Density \u003d WEIGHT
VOLUME

From the course of mathematics, you know that the value of any fraction shows the number of units of a quantity in the numerator per one unit of the quantity in the denominator. The density of a substance is also a fraction value. therefore the numerical value of the density of a substance indicates the mass per unit volume of this substance. For example, the density of cast iron is 7 kg / dm 3. This means that 1 dm 3 of cast iron has a mass of 7 kg. The density of fresh water is 1 kg / l. Therefore, the mass of 1 liter of water is 1 kg.

The density of a substance is expressed very often and in grams per cubic centimeter

Consider the density table on the page of the textbook 50-51
- Determine the substances with the highest density from the table? The lowest density?
- The density of aluminum is 2700 kg / m 3. What does this number mean?
- Express in kilograms body mass: 2.5t; 0.25 g; 300 g
- The density of tin is 7.3 g / cm 3 and the density of granite is 2600 kg / m 3. Which substance has a higher density?

Should remember that the density of the same substance in a solid, liquid and gaseous state is different
ice density
density of water
water vapor density

AREOMETER (or otherwise densitometer)
This is a device in the form of a glass float with a measuring scale and a load, designed to measure the density of liquids and bulk solids.

Formulas can be transformed according to the rules of mathematics. Therefore, the density formula can be written in two other forms:

DO YOU KNOW
Based on the data from deep surveys of galaxies, one can also determine the average density of matter in the Universe. Today, the data of such calculations indicate that, on average, outer space is extremely rarefied.
If you mentally uniformly “smear” matter throughout the entire volume of our Galaxy, then the average density of matter in it will be approximately equal
0,000,000,000,000,000,000,000,000 5 5 g / cm 3.

DO YOU KNOW
The earth's crust consists of layers of matter of varying density. The average density of the earth's crust and the Earth as a whole are, respectively, 2700 and 5520 kg / m3.
Since the main person consists of liquid, the average density of a person’s body is 1 g / cm3 or 1 kg / l. From this it follows that the mass of a person in kilograms is numerically equal to the volume of his body in liters. For example, a student weighing 50 kg has a body volume of about 50 liters. Such a volume of water will be on the floor when immersed in a bathtub filled to the brim with water.
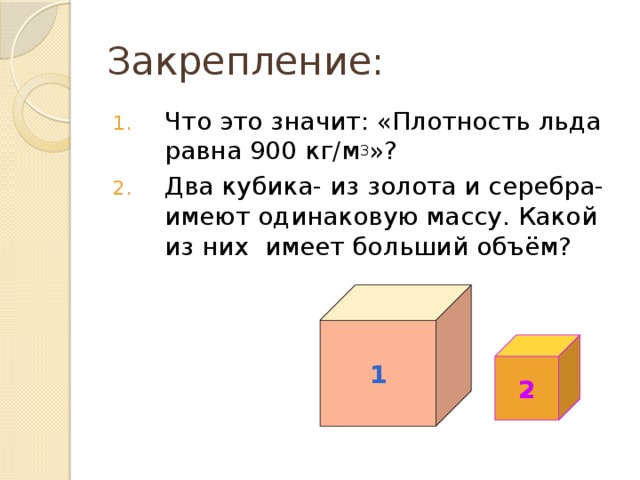
Fastening:
- What does this mean: “The density of ice is 900 kg / m 3”?
- Two cubes - from gold and silver - have the same mass. Which one has the larger volume?

Fastening:
- The mass of each of the bodies is 1t. Which one has less density?
- Which of the two bodies has a higher density?
- Three cubes - made of marble, ice and brass - have the same volume. Which one has the largest mass, which is the smallest?
- Will the density of plasticine change if a piece of plasticine is rolled into a ball? Why?
brass
marble

Answer: those who are interested in the density of a substance want to know what is the mass of one cubic meter or cubic centimeter of this substance. The weight density of 0.025 g / cube 3.
this is the density of foam rubber. Make yourself a foam weight, paint it with black paint and slowly squeeze out five times every morning. Just don’t forget to groan. Mom will be shocked.
- In the circus, a clown with one left lifts a huge kettlebell, on which is written 500 kg. In fact, the mass of the weight is one hundred times less. The volume of this weight is 0.2 cubic meters 3. Calculate the density of the circus kettlebell.

At the chemist's birthday, the physicist was treated with two cutlets. One lamb cutlet with garlic, the other of plasticine with small nuts. How do these two cutlets differ from the point of view of a physicist? What is the reason from the point of view of physics that these cutlets have the same shape and volume, but different masses?
Answer: any physicist immediately distinguishes the taste of a meat patty from plasticine. Moreover, one with garlic and the other with nuts. If we argue from the point of view of physics, the substances from which these cutlets are molded have a different average density, therefore, with the same volumes, the masses are different.

6. The sad uncle Borya wanted to cook soup for himself, and he turned out half a pan of green muck. Volume this muck that Uncle Borya did not dare to try - 0.001 m 3 , weight - 1 kg 300g. Calculate density uncle Borin filth.
Answer: the density of green muck, which the sad Uncle Borya never could tear from the pan, 1.3 g / cm 3.

7. The mass of the empty bottle is 450 g. The mass of the same bottle filled with water is 950 g. And the mass of the bottle filled with the bitter sour meat that the doctors prescribed the sad Uncle Bore to drink 3 times a day before meals, 980 g. Knowing the density of water - 1 g / cc, determine, without grimacing, the density of this healing sour meat, which Uncle Borya drinks with disgust 3 times a day.
Answer: the density of sour meat is 1.06 g / cm 3. Uncle Borya whips her with a very sour expression on her face.

Reiteration:
- How can one find the density of a substance?
- What letter denote density?
- What is the unit of density in SI?
- What other density units do you know?

Homework:
- §21, exercise 7 (1 - 3)
Date:
Subject: Mass and density of matter.
Purpose:give the concept of mass and density of matter
Tasks:
Educational: student education knowledge of the mass and density of a substance.
Educational : carry out moral education, respect for people.
Developing : develop oral speech, attentiveness, the ability to work with the source.
Lesson type: combined lesson
During the classes
Organizing time
Checking homework:
What thermal phenomena do you know? What is body temperature?
Is diffusion related to body temperature? Why?
What aggregate states are substances in? What explains their transition from one state to another?
Describe the distinctive properties of solids?
Describe the distinctive properties of liquids?
What are the properties of gaseous substances and their distinctive features?
New topic
Nature is designed in such a way that any body necessarily interacts with any otherbody. An example of this is the collision of two bodies, as well as the interaction
bodies connected by a spring or thread. Observation of the movement of bodies shows thatthe effect of bodies on each other is bilateral
, i.e., has the nature of interaction.
Interaction is a complex phenomenon. Let's consider the simplest examples.interactions that we observe in everyday life. Later, summarizing the knowledge gained, we will consider more complex types of interaction.
Suppose one of the boys on the rink made a push of his handmove another. At the same time, he himself will roll back. At the same time, the smaller boy will move faster than the older and larger boys.
If the running and slow walking boys collide, thenboth of them will also change their speeds.
Working with oars, a person interacts with water. As a result, the boat moves forward, and the water pushes back.
The given examples indicate that the action of bodies on each other is two-sided and has the character of interaction. So, it turns out not an action, but an interaction.
So, the reason for changing the speed of a body is always its interaction with other bodies. Moreover, the speed of the bodies varies in different ways. If a body, when interacting with another body, changes its speed less, then they say that itmore inert. This means that every body has the ability to resist changing its current state.
For a quantitative comparison of the inertia of different bodies, a physical quantity calledmass.
So, mass is a measure of the inertia of bodies. It is denoted by the lettert
In practice, it is more convenient to find body weight not by its interaction with another body, but by weighing it on the scales. The principle of weighing on a lever scale is balancing. Using different weights, balance is achieved. In a state of equilibrium, the weight of the body is equal to the mass of weights. Smaller units are also used to measure mass.
To measure the mass of any body, it must be compared with a body whose mass is taken as a unit.
In the International System of Units (SI), a kilogram (kg) is taken as a unit of mass. This is the mass of a reference cylinder weight, cast from an alloy of platinum and iridium. The international kilogram standard is stored in the museum of standards in France in Sevres, near Paris. Many countries have exact copies of this standard. For use in everyday life, a set of weights of different weights is made, calledby weight. The principle of weighing on a lever scale is balancing. Using different weights, balance is achieved. In a state of equilibrium, the weight of the body is equal to the mass of weights. To measure the mass use also smaller units of mass - the thousandth and millionth of a kilogram -gram (g) andmilligram (mg) and for weighing a body with a large mass - larger units -centner (c) andton (t).
1 t \u003d1000 kg;
1 r \u003d0,001 kg;
1 mg \u003d0,000 001 kg
All bodies of the environment are composed of any substances: wood, iron and many others. All bodies have a certain shape, volume, area, mass, etc.
If necessary, we can determine such characteristics of bodies using measurements and calculations. Body weight can be determined by weighing on a scale. It depends on the size of the body and the substance from which it is made. The mass of bodies made from various substances will be different. For example, 1 m iron 3 has a mass of 7,800 kg, and lead of the same volume - 13 thousand kg. By comparing bodies having the same volume, we can determine which one is heavier and which is lighter. This implies the need to introduce a physical concept characterizing the property of a substance calleddensity.
Substance density called a physical quantity numerically equal to the mass per unit volume of this substance.
If we denote the density of the substance by the letter p (the Greek letter "ro"), the body mass ist body volume -V then the density of the substance is determined by the formula
In the International System of Units (SI), the density unit is takenkilogram per cubic meter (kg / m 3 ). This is the density of a homogeneous substance, the mass of which 1 kgwith volume 1m 3 .
Fastening:
1. What is mass?
2. What is the density of a substance?
3. What is the formula for finding the density of a substance?
5. Reflection.
6. Homework: p. 31-32
























![]()











 Back forward
Back forward
Attention! The slide preview is used for informational purposes only and may not give an idea of \u200b\u200ball the presentation features. If you are interested in this work, please download the full version.
Slide No. 1 “Cover”
Slide №2 "Title"
Objectives:
- educational - to teach to determine the dependence of body weight on the type of substance and on the volume of the body; give the concept of the density of matter, its units of measurement; give the concept of the physical meaning of density; teach you how to use the density table; to develop skills for applying knowledge in specific situations;
- developing - to develop the ability to think, analyze, generalize; to develop students' speech through the organization of dialogic communication in the lesson; to develop practical labor skills, to form feelings of novelty and curiosity; to develop independence, creativity, horizons;
- educating - to educate attention, diligence, hard work, accuracy and the ability to organize your workplace, the desire to achieve the goal.
Lesson type - combined, i.e., a lesson of generalization and systematization of knowledge with elements of explanation of new material.
Type of lesson: lesson of non-traditional form - a lesson-journey.
Teaching methods:
- dialogic (with verbal feedback),
- problematic (creating a problematic situation),
- partial search (incentive to solve a problem situation using search activity),
- practical (conducting experimental experiments, solving exercises);
- active learning method - imitation (game).
Applied training technologies:
- computer (throughout the lesson);
- gaming technology (throughout the lesson);
- technology of problem education (at the stage of the formation of new ZUNs);
- group technology (throughout the lesson);
- test (at the stage of updating knowledge and consolidating new ZUNs).
Equipment:
- computer, projector, screen
- electronic scales - 6;
- beakers - 6
- table number 1 on the format A3 - 6;
- table No. 2 in A4 format - 6;
- black felt-tip pens - 6
- small cylinders - 6
- large cylinders - 6;
- coasters with a name and logo for each group.
Students are divided into 6 groups of 5 people.
DURING THE CLASSES
I. Organizational moment(8 min.)
1) Mobilizing beginning (readiness for lesson - 2 minutes)
Slide number 3.
Teacher: Today we have an unusual lesson - travel, and for its implementation you are divided into groups (teams). You will travel (click on slide No. 3 “School” - the cursor will automatically show the path) at the following stations: “Repeat”, “Problem”, “Explanatory”, “Crossword”, “Tablichnaya”, “Poreshaikina”, “Test”, going to the Palace of Knowledge. Each group will move under its own name: “Diligent”, “Inquisitive”, “Persistent”, “Purposeful”, “Inquisitive”, “Diligent”.
On the tables on the stands are the emblems of the teams and their names.
2) Actualization of knowledge (6 min.)
Teacher: We set off on a journey. Let's start the lesson with repetition. What was the name of the past topic?
Pupils:Phone tel. The mass of the tel.
Teacher: Repeat the topic using tests. The first stop at the station "Repeat" (click on the article “Repeat” - the team emblems automatically move to this station).
Slide number 4.
Teacher:To do this, you have computers on the tables. (Each group has its own computer on which the team performs the test). The time to complete the test is programmed, and a mark for correctness will appear automatically.
There is a frontal survey using test technology and ICT, using the program "Macromediaflesh». (Annex 1 )
NOTE: To conduct a survey, you must first install the Macromedia flesh program.
Slide number 5 Teacher:Everyone did a good job at this station, no one fell behind. We keep on going to the station. "Problem" (click on the article “Problemnaya” - the team emblems move).
II. Formation of new ZUNs(26 min.)
Slide number 6.
1) Motivation (5 min.)
Teacher:What appliances do you see on your tables?
Pupils:Beakers and scales.
Teacher:What can be determined using these instruments?
Pupils: The volume of bodies and their mass.
Slide number 7.
Problem learning technology . Formulation of the problem.
Teacher: (click on the slide - a question appears). If we determine the volume of the body and its mass, then what problem will we solve today?
Pupils: We reveal the correspondence between the mass and volume of bodies.
Teacher: At the Problemnaya station, each group is assigned a task (problem): after determining the mass and volume of the body, you must fill out the table and draw a conclusion.
On the tables in each group are 2 bodies of different sizes, but made from one substance. Pupils take measurements and fill out table No. 1 (on tables) with felt-tip pens and conclude that the ratio of body weight to its volume for two given bodies turned out to be the same.
Slides number 8-9.
2) Formation of new ZUNs (21 min.)
Teacher:You yourself have seen that for a given substance, the ratio m/ V \u003d const.
This value is called the DENSITY of the substance and is denoted by the Greek letter ρ (po). ρ - the density of the substance.
The formula for determining the density:
What is the relationship between density and mass? Between density and volume?
Student: Density is directly proportional to mass. The greater the mass of the body, the greater its density. Density is inversely proportional to body volume. The larger the volume of the body, the lower its density.
Teacher: (clicking on a slide - a density determination appears).
Density determination: Density is physical. a value equal to the ratio of body weight to its volume.
Teacher: Who can deduce the units of density in the SI system?
Student: Because the mass in SI is measured in [KG], and the volume in [M 3], the units of density in SI will be [kg / m 3].
Teacher: We write ρ \u003d [kg / m 3].
Sometimes density is measured in [g / cm 3].
Since the units of measurement can be both those and others, then there must be a correlation between them.
1kg / m 3 \u003d ... g / cm 3. Your homework will deduce this ratio.
From the density formula we make a wonderful triangle: 
How to determine the mass or volume from it?
Student: m \u003d ρV; V \u003d m / ρ.
Teacher:The density of this substance is a constant value, and, as follows from the formula, characterizes the mass of a body in a unit of its volume. The values \u200b\u200bof different densities are listed in tables No. 2, 3, 4, which you have in the textbook on pages 50, 51 and in the book of V.I. Lukashik.
See tables for solids, liquids, and gases. All data are entered at a certain temperature t \u003d 20 o C. And how will these data change if the temperature, for example, increases?
Student: With increasing temperature, the molecules of the bodies begin to move faster, and the volume of the body increases. And as the body volume increases, then its density decreases.
Teacher: Let's learn how to work with a table. Determine the density of iron.
Student:ρ iron \u003d 7800 kg / m 3.
Teacher: What do you think this means? Slide number 10.
Student:The mass of 1 m 3 of iron is 7800 kg.
Teacher:Wonderful. We leave for the station “Explanatory”. (Click on the article “Explanatory” - the emblems moved).
Slides No. 11-12.
Pupils should, using the density table, find the given density of the substance and explain its value, for each team its own value is given.
Teacher:Everyone did a good job at the Explanatory station. Moving on to the station. "Crossword". (Click on the article “Crossword” - emblems at this station).
Teacher: Let's practice the table and play - guess the crossword puzzle. Stop at the station "Crossword".
Slides No. 13-14.
Questions to the crossword puzzle:
When you click on a question number, an answer automatically appears.
- Which solid has the minimum density? (Bung)
- The density of this gas is the highest. (Chlorine)
- Which gas has the smallest density? (Hydrogen)
- The density of this fluid is the highest. (Mercury)
- The density of this solid is the same as that of ice. (Paraffin)
- The density of this substance \u003d 800 kg / m 3 (Kerosene)
- The density of this substance is the highest in the table. (Osmium)
- The density of this substance \u003d 19300 kg / m 3 (Gold)
- The density of this substance is the same as that of alcohol. (Oil).
Teacher: What keyword did we get? (Density).
We’ll work again. They took several bodies made of different substances, and determined their masses and volumes. Found that the masses and volumes were the same. What substances can these bodies be made of?
1) kerosene, alcohol, oil;
2) engine oil, ice, paraffin;
3) ether, gasoline;
4) marble, aluminum;
5) sea water, whole milk;
6) concrete, porcelain.
Teacher: If you take the same substance in different states. Will its density be different?
Student: Yes, because the molecules are located differently in different states of matter.
Teacher: Take water. Find its density in various states. And why is the density of ice less than the density of water?
Student: Because ice molecules are arranged in a strict order, they have a crystalline structure.
Slide number 15.
Teacher: Everyone did a good job at Krossvordnaya station. Still refueled with knowledge. (Click on the article “Tablichnaya” - emblems at this station). Moving on to the station. Tabular.
Slides number 16-17.
We will exercise more. Assignment for rups: using the formula for calculating the density, fill in table No. 2. Each group has its own task.
After filling, the calculations are verified on the screen (by clicking, empty cells are filled).
III. Material fastening(8 min.)
Teacher:Wonderful knowledge showed all the teams. We keep on going to the station. "Poreshaykina" (click on the article "Poreshaykina" - emblems at this station).
Pupils, using the "Collection of Problems in Physics" by V.I. Lukashik, consolidate their knowledge. Each group is given 1 task.
Slides number 18-19.
№ 228. Two cubes of the same mass are given: one from amber, the other from copper. Which of the cubes has a substance mass of 1 cm3 more and how many times?
Slide number 20.
№ 229. Of the two copper rivets, the first has twice as much mass as the second. What is the ratio of the volumes of these bodies?
№ 230. The diameters of copper and aluminum balls are the same. Which one has less mass and how many times?
Slide number 21.
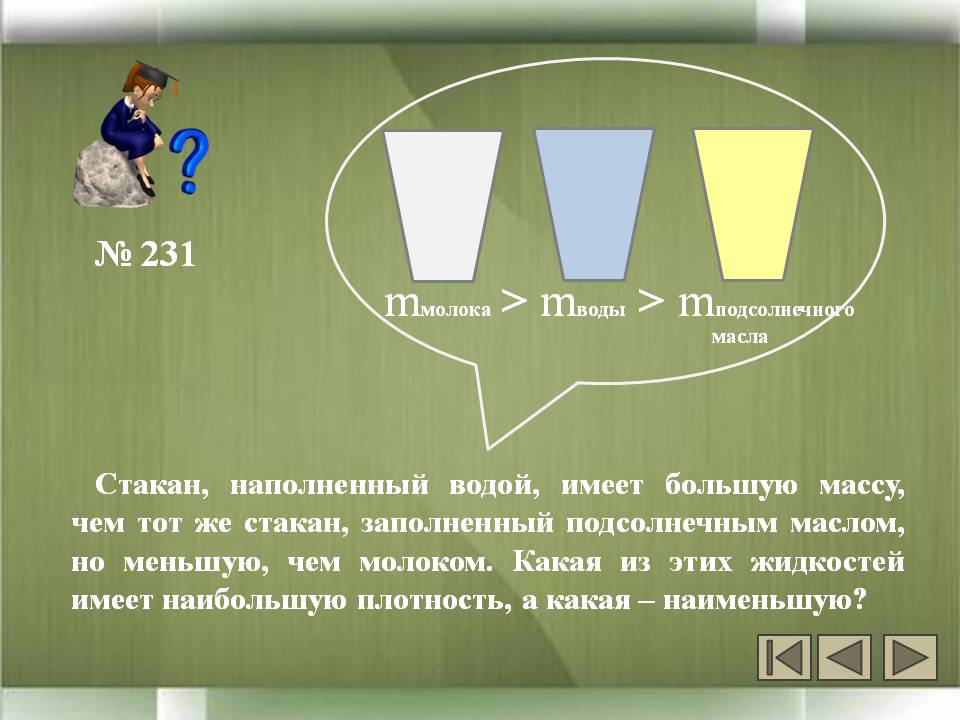
№ 231. A glass filled with water has a larger mass than the same glass filled with sunflower oil, but smaller than milk. m milk \u003e m water \u003e m sunflower oil Which of these fluids has the highest density, and which is the smallest?
Slide number 22.
№ 232. On the cups of balanced weights are cubes. Are the densities of the substances the cubes are made of?
№ 233. Water was poured into one vessel from two identical vessels, and sulfuric acid of equal weight was poured into the other. What liquid has a large mass?
Slides No. 24-23.
Teacher: Here are some interesting facts.
1. The average density of the Earth \u003d 5500 kg / m 3
2. The average density of the moon \u003d 3300 kg / m 3
3. The average density of a person \u003d 1036 kg / m 3. How would you define it?
4. In Italy, near the city of Naples, there is a "Dog Cave", in the lower part of which carbon dioxide is constantly emitted. For whom, human or dog, is this cave safe?
Teacher: Now, on the way to the Palace of Knowledge, we still have the last Testovaya station (click on Art. Test - emblems at this station).
Slide number 25.
Answers to test questions are automated: with the correct answer, “TRUE” appears, if the answer is incorrect, “WRONG”.
Tests are given on slides No. 26-30.
Teacher: (click on the Palace of Knowledge - emblems moved there). You went through all the stations on the way to the Palace of Knowledge and replenished your knowledge base. The palace will open the doors for you and give you the highest marks. And you will return there more than once.
Slide number 31.
V. Lesson summary(2 minutes.)
Slide number 32.
VI. Homemade the task(1 minute.)
§ 21, exercise 7;
IN AND. Lukashik "A collection of problems in physics"
№ 234-237;
withdraw 1kg / m 3 \u003d ... g / cm 3

